
FIL at a glance..
A congenital (born with) overgrowth condition affecting one half of the face. Ranging from mild to severe. Caused by activating mutations in the PIK3CA gene. The mutation happens at random during early development, not every cell is affected and there are no known cases of inheritance. Only rarely is the mutation found in blood so testing should be done on affected tissue. There is sadly no cure for FIL and a high risk of regrowth with surgery. Advances made in precision medicine is creating hope for treatments.


Characteristics of Facial Infiltrating Lipomatosis
The most common features, affecting only one side of the face, are:
Please note that not all patients with FIL have all these symptoms. FIL can range from mild to severe.
Visit our FAQ page for answers to the most frequently asked questions about Facial Infiltrating Lipomatosis.
Multible names
All of the above are some of the names being used for the same ultra-rare craniofacial overgrowth
syndrome. One differential diagnosis is Congenital Hemifacial Hyperplasia (previously called
Hemifacial Hypertrophy), but for this diagnosis the facial asymmetry remains consistent without
progressive growth and without the infiltrative lipocytes (fat cells).
The condition was first described in 1983 by Slavin and colleagues and about 60 reported cases of FIL have been found in literature. Though ultra-rare (by EU definition less than 1 in 50 000) the prevalence of FIL is likely underestimated as there are multible terminologies for the condition, many undiagnosed, misdiagnosed, those who have not been published in medical literature and those where FIL is only one part of their syndrome. The condition seems to affect both genders equally and is seen among different ethnicities.
FIL can be an isolated condition or associated with other conditions like M-CM, Hemimegalencephaly or CLOVES syndrome which are also a part of the PIK3CA Related Overgrowth Spectrum (PROS). You can read more about PROS on our PROS page.
Diagnosis
A diagnosis of FIL can be based solely on clinical observations, but Magnetic Resonance Imaging (MRI) provides the best delineation.
FIL is a diagnosis on a spectrum and to precisely differ between the variations can sometimes be difficult. Some doctors may not want to make a clinical delineation between the different entities on the spectrum and instead diagnose patients with the umbrella terms like Segmental Overgrowth Syndrome or PROS Syndrome. Each of the conditions on the spectrum brings with them a set of very different issues. Due to the rarity of these conditions connecting with others with the same condition is important. Most doctors you meet will never have heard of these conditions before let alone treated it. WonderFIL smiles aim to bridge the gap and can lead you towards doctors with FIL experience.
If you do not have any experienced doctors near you, Boston Children’s Hospitals Vascular Anomalies Center does case reviews each week in conference meetings. They review medical history, look at photographs, radiographic images and pathology slides sent in by referring physicians or families from across the United States and around the globe. Based on this information, the team provides diagnoses and treatment recommendations, and answers specific questions posed to them by physicians and families, without the patient having to travel to Boston.
Genetics
The cause of Facial Infiltrating Lipomatosis is a random, non-inherited mutation in the PIK3CA gene located on the long arm of chromosome 3. Several rare overgrowth and malformation conditions are caused by mutations in this gene. The mutation occurs in the early stages of development. Why this mutation spontaneously occurs is not known. The most common mutation in FIL is a missense mutation, which is basicly a copying error. One of the four DNA nitrogenous bases (A,T,C,G) is changed in one cell and all cells derived from this mutated founding cell will carry this mutation. Giving the person cells with two genotypes, cells with the mutation and cells without. It's called genetic mosaicism. So not every cell is affected, but theoretically cells with this mutation may influence non-mutated cells nearby.
The mutation causes a different amino acid to be inserted into the protein. In FIL (/PROS) this change causes an overactivity, a gain of function. For people with isolated FIL the cells that carry the mutation are restricted to one half of the face and/or skull. Timing of the mutation, specific location on the gene (mutation variant) and cell type may play a part in how much is affected and the severity.
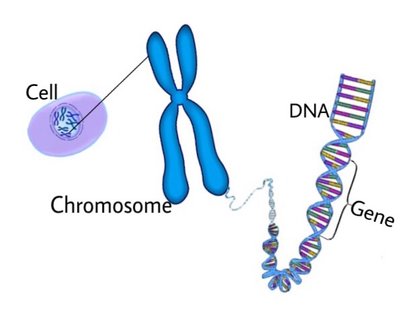
Below is an illustration of a gene without and one with a missense mutation. The nucleotide Adenine, which should be base paired with Thymine, is changed into Guanine (which should be paired with Cytosine). RNA bases are in groups of three and this is how amino acids are coded for. In the example below it reads the amino acid H (for Histidine), but in the one with the mutation it is A (for Arginine).
If we call DNA the recipe, then RNA is what brings the recipe to life.
Genetic Testing
You would usually not find the mutation in a blood sample and due to the mosaic nature genetic testing must be preformed correctly. The mutation can be confirmed by doing a Next Generation DNA sequencing (NGS) as standard sequencing is ineffective at detecting changes that only affects a small percentage of cells. Testing should be done on affected tissue taken by puncture biopsy (or collected from surgery). For FIL patients a non-invasive buccal swab, also called a cheek-swab have shown a high diagnostic sensitivity similar to detection rate in tissue biopsi. Other testing options for FIL patients are testing on shed teeth or gingival (gum) biopsy. The latter option can be done as a "brush biopsy" which is non-invasive. Since the mutation is mosaic a negative result does not necessarily rule out a clinical diagnosis on the PIK3CA Related Overgrowth Spectrum.
The significance of a confirmed mutation is espicially due of the rising
potential of pharmacological intervention.
With pharmacological intervention meaning targeted therapy, also called precision medicine, and present and upcoming clinical trials where you’ll need a documented mutation in order to be considered for treatment.
Canser risk
PIK3CA is an oncogene (a gene that has the potential to cause cancer) that plays an important role in tumor progression, but it does NOT seem that FIL patients are more prone to cancer. There is however a need for collection of data and research about the risk. In one article 4 out of 122 patients with the PROS condition CLOVES had been diagnosed with Wilms’ tumor (also known as nephroblastoma - a rare kidney cancer). On the basis of this article quarterly abdominal scans up till the age of 8 (when the risk reduces) has been recommended. No FIL patients have been reported to have had Wilms’ tumor.
Treatment options
There is currently no cure for FIL and treatment options are few. Recently (April 2022)
FDA approved Novartis Vijoice® (alpelisib) as first and only treatment for select patients with PIK3CA-Related Overgrowth Spectrum (PROS) conditons.
Other options are surgical with a very high risk of recurrence after surgery (79% chance reported in article review) and/or drug therapy. Surgical removal of the fatty tissue is often difficult due to it infiltrating important facial structures.
The advances made in targeted therapy is creating hope.
The PIK3CA inhibitor Alpelisib (now marketed under Vijoice) have shown remarkable results in a (non clinical) study. “During 6 months of treatment, all patients showed substantial clinical improvement”. One of the authors of the article, Dr.Canaud, held a speech about the results at the ESHG 2019. You can watch it here (from 57:00). The clinical trial for Alpelisib treament in PROS patients had it's study start date April 19th 2021.
The current drug treatment options are targeted therapeutic drugs in the
PI3K/AKT/mTOR signalling pathway. There are also several single, dual
and multi-target inhibitors for this pathway currently in development for
the treatment of several different forms of cancer.
Below is a description of the drug options currently available upon eligibility.
A simplified illustration of the PI3K/AKT/mTOR signaling pathway. A crucial pathway for cell survival, angiogenesis (development of new blood vessels) and proliferation (reproduction). Growth factor (i.e. insulin) activities PI3K, which will further activate AKT, which will in turn activate mTOR. mTOR influences diverse transcription factors that finally leads to the expression of the gene products (growth, angiogenesis, proliferation). Included in the illustration are the above mentioned inhibitors and where they target in the pathway.
Alpha-specific PI3K (PIK3CA) inhibitor
Generic name: Alpelisib Brand Name: Vijoice (or Piqray for breast cancer indication). Previously known as BYL719
On april 6th 2022 FDA approved Novartis Vijoice® (alpelisib) as first and only treatment for select patients with PIK3CA-Related Overgrowth Spectrum (PROS) conditons. An approved treatment to specifically address the root cause of PROS conditions in select patients 2 years of age and older. Currently only FDA approved.
Alpelisib was FDA approved under the brand name Piqray the summer of 2019 for the treatment of HR+/HER2- advanced breast cancer (in combination with a fulvestrant). Approved by the EC and Health Canada summer 2020.
A (non clinical) study was published in the science journal Nature in June 2018 about a cohort of 19 PROS patients who all demonstrated clinical improvements and minimal side effects by this treatment (long term side effects still unknown). This drug is for some available through compassionate use program/managed access program, for those who are eligable. A clinical trial , named EPIK-P2, for PROS patients started recruitment April 19th 2021. A retrospective study of PROS patients who have received
Alpelisib as part of a compassionate use program started June 9th with completion date April 16th 2021.
pan AKT-inhibitor
Generic name: Miransertib Name: ARQ-092 / MK-7075
This drug is currently in clinical trial for the treatment of both PROS and Proteus Syndrome (another overgrowth condition caused by AKT1 mutation). Interim data from phase 1/2 of this trial showed a manageable safety profile and the majority of patients demonstrated no disease progression. This trial is still open for recruitment. An article published February 2021 on the use of this drug in two children (one with FIL) can be found here. Both patients discontinued the use of
Miransertib to start Alpelisib.
mTOR-inhibitors
Generic name: Sirolimus Brand Name: Rapamune / Rapamycin (exists as both oral and topical)
Generic name: Everolimus Brand Name: Afinitor / Votubia / Zortress (US) / Certican (EU +) / Evertor
Sirolimus is a drug indicated for posttransplant immunosuppression, but there has been off-label(*) use of this drug for patients with overgrowth diseases for several years. The conclusion from a recent study of Rapamycin in PROS patients: “This study suggests that low-dose sirolimus can modestly reduce overgrowth, but cautions that the side-effect profile is significant, mandating individualized risk–benefit evaluations for sirolimus treatment in PROS.”
A related drug to Sirolimus, also an mTOR inhibitor, is everolimus, marketed under different brand names for different indications but they have the same active ingredient. Everolimus is also being used off-label for patients with overgrowth conditions.
*Off-label is the use of a drug for a condition other than that for which it has been officially approved for.
Clinical Trials
Managed Access Program
Study opportunities
PIK3CA Related Overgrowth Spectrum - PROS
Gain of function mutations in the PIK3CA gene can cause a wide range of rare malformation and overgrowth disorders. These conditions have been gathered under the collective umbrella term PIK3CA Related Overgrowth Spectrum (PROS). Some may have phenotypic features that clearly falls into one of the entities, but for others clinical delineation can be difficult as features can be overlapping. This leaves some to only receive a diagosis of PROS, sometimes then referred to as PIK3CA Related Overgrowth Syndrome. The mutation is only rarely detected in a bloodtest. Testing should be done on tissue samples from the overgrowth area.
You can read more about PROS here.

Articles about Facial Infiltrating Lipomatosis - FIL
For articles related to the PIK3CA mutation and more, visit the About PROS page.
1983 - Congenital Infiltrating Lipomatosis of the Face: Clinicopathologic Evaluation and Treatment
1989 - CONGENITAL INFILTRATING LIPOMATOSIS
2004 - Congenital Infiltrating Lipomatosis of the Face and Neck
2009 - Case Report Congenital infiltrating lipomatosis
2010 - Facial infiltrating lipomatosis
2011 - Facial infiltrating lipomatosis: A case report and review of literature
2012 - Congenital diffuse infiltrating facial lipomatosis
2012 - Case Report Congenital infiltrating lipomatosis
2013 - Infiltrating lipomatosis of the face: A case series
2014 - PIK3CA activating mutations in facial infiltrating lipomatosis
2015 - Congenital infiltrating lipomatosis of face: Case report and review of literature
2017 - Somatic PIK3CA mutations are present in multiple tissues of facial infiltrating lipomatosis.
2018 - Congenital Infiltrating Lipomatosis of the Face: Case Report and Literature Review
This website is intended to connect families and people with Facial Infiltrating Lipomatosis (FIL), share experiences, provide information and support.
It is not intended for medical diagnostic use or to replace medical consultation. No images on this website may be used or reproduced.
Copyright © 2024 WonderFIL smiles. All rights reserved. WonderFIL smiles is a registered non-profit organization.